A fluorogenic monolayer to detect the co-immobilization of peptides that combine cartilage targeting and regeneration†
Abstract
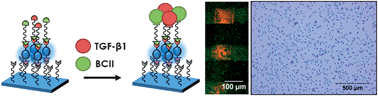
Strategies to generate platforms combining tissue targeting and regeneration properties are in great demand in the regenerative medicine field. Here we employ an approach to directly visualize the immobilization of cysteine-terminated

 Please wait while we load your content...
Please wait while we load your content...