Synthesis of mesoporous LiMn2O4 and LiMn2−xCoxO4 thin films using the MASA approach as efficient water oxidation electrocatalysts†
Abstract
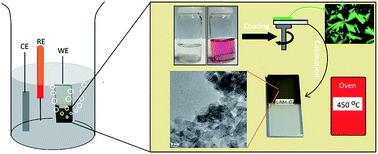
Mesoporous, highly active, robust, and cost-effective thin films are in big demand for water splitting by electrocatalysis. Molten-salt assisted self-assembly (MASA) is an effective method to synthesize mesoporous thin films. Transparent clear solutions of salts (LiNO3 and [Mn(H2O)6](NO3)2), acid (HNO3), and surfactants (CTAB and P123) can be spin-coated over substrates as liquid crystalline (LC) films and calcined to obtain mesoporous high quality transparent thin films. A mixture of three salts (LiNO3, [Mn(H2O)6](NO3)2, and [Co(H2O)6](NO3)2) also forms LC mesophases that can be calcined to produce mesoporous nanocrystalline mixed metal lithiates (meso-LiMn2−xCoxO4) with surface areas as large as 144 m2 g−1 (for LiMn1.5Co0.5O4). The synergic effects of these salts improve the pore-size of the final products; the pore size drops from around 11 nm (in the meso-LiMn2O4) to 6–7 nm in the meso-LiMn1−xCoxO4. The meso-LiMn2−xCoxO4 films were tested at pH 13.6 as water oxidation electrocatalysts over a broad range of x. While meso-LiMn2O4 shows a low activity towards water oxidation, the catalytic activity increases with the increasing Co(III) content of the films. The highest mass activity per cobalt, 1744 A g−1, is obtained for meso-LiMnCoO4, which remains as a robust and efficient film even at a current density of 120 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...