Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium†
Abstract
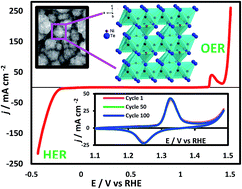
Designing efficient electrocatalysts has been one of the primary goals for water electrolysis, which is one of the most promising routes towards sustainable energy generation from renewable sources. In this article, we have tried to expand the family of transition metal chalcogenide based highly efficient OER electrocatalysts by investigating nickel telluride, Ni3Te2 as a catalyst for the first time. Interestingly Ni3Te2 electrodeposited on a GC electrode showed very low onset potential and overpotential at 10 mA cm−2 (180 mV), which is the lowest in the series of chalcogenides with similar stoichiometry, Ni3E2 (E = S, Se, Te) as well as Ni-oxides. This observation falls in line with the hypothesis that increasing the covalency around the transition metal center enhances catalytic activity. Such a hypothesis has been previously validated in oxide-based electrocatalysts by creating anion vacancies. However, this is the first instance where this hypothesis has been convincingly validated in the chalcogenide series. The operational stability of the Ni3Te2 electrocatalyst surface during the OER for an extended period of time in alkaline medium was confirmed through surface-sensitive analytical techniques such as XPS, as well as electrochemical methods which showed that the telluride surface did not undergo any corrosion, degradation, or compositional change. More importantly we have compared the catalyst activation step (Ni2+ → Ni3+ oxidation) in the chalcogenide series, through electrochemical cyclic voltammetry studies, and have shown that catalyst activation occurs at lower applied potential as the electronegativity of the anion decreases. From DFT calculations we have also shown that the hydroxyl attachment energy is more favorable on the Ni3Te2 surface compared to the Ni-oxide, confirming the enhanced catalytic activity of the telluride. Ni3Te2 also exhibited efficient HER catalytic activity in alkaline medium making it a very effective bifunctional catalyst for full water splitting with a cell voltage of 1.66 V at 10 mA cm−2. It should be noted here that this is the first report of OER and HER activity in the family of Ni-tellurides.


 Please wait while we load your content...
Please wait while we load your content...
