In3+-doped BiVO4 photoanodes with passivated surface states for photoelectrochemical water oxidation†
Abstract
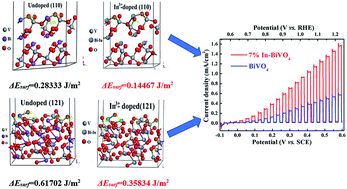
BiVO4 is a promising photoanode material for photoelectrochemical water splitting, but its actual activity is hindered by the high energy surface states. Here, we report that In3+ can be used as a dopant to substitute the partial sites of Bi3+ in BiVO4 for modifying the surface states and improving the water oxidation activity of a BiVO4 nanoflake film. Among the In3+-doped BiVO4 film photoanodes, the 7% In3+-doped BiVO4 film shows optimal photoelectrochemical water oxidation activity. At 1.23 V vs. RHE, the 7% In3+-doped BiVO4 photoanode exhibits a photocurrent density of 1.56 mA cm−2 in 0.1 M Na2SO4, which is over 200% greater than that of the undoped BiVO4 photoanode. In3+-doping did not change the morphology, phase and band gap of BiVO4 obviously, but resulted in a positive shift of the flat band position and higher surface charge separation efficiency for water oxidation. Density functional theory calculations indicate that the surface energy of BiVO4 decreased after In3+-doping that involved more unsaturated electrons of the Bi atom in the Bi–O bonds, thus reducing the amount of exposed unsaturated Bi atoms and broken Bi–O bonds. Therefore, the enhanced water oxidation activity on the In3+-doped BiVO4 photoanode can be ascribed to In3+-doping that passivated the surface states of BiVO4 and thus inhibited the surface charge recombination.


 Please wait while we load your content...
Please wait while we load your content...