Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets†
Abstract
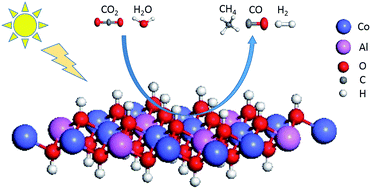
The photoreduction of CO2 to carbon energy sources over catalysts is of great interest and significance. However, though many studies have been conducted at high CO2 concentrations, research on CO2 photoreduction at atmospheric concentrations remains rare. Herein, CoAl-layered double hydroxide (CoAl-LDH) nanosheets have been synthesized as a platform to explore low-concentration CO2 photoreduction. The CoAl-LDH nanosheets exhibited efficient photocatalytic activity for CO2 photoreduction to CH4 at atmospheric concentration (400 ppm) under simulated solar light, and the reaction proceeded without deactivation for 55 hours. A CH4 production rate of 4.3 μmol g−1 h−1 was achieved without the assistance of any sacrificial agent or noble metal. CO2 adsorption experiments showed that the CoAl-LDH nanosheets exhibited an adsorption capacity of 2.95 cm3 g−1, which was about twice that of P25 with a comparable specific surface area. XPS analysis indicated that CH4 generation was closely related to the divalent cobalt, and the CH4 yield decreases sharply when the divalent cobalt is oxidized, which was ascribed to the dissociation of water to release H for the hydrogenation of intermediates through further experimental analysis. This work emphasizes the importance of the alkaline OH group for the efficient adsorption of low-concentration CO2 and reveals the unique property of divalent cobalt for CO2 photoreduction.


 Please wait while we load your content...
Please wait while we load your content...