Chemical strain formation through anion substitution in Cu2WS4 for efficient electrocatalysis of water dissociation†
Abstract
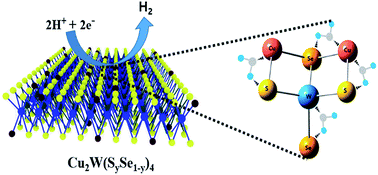
Researchers have revealed that the electrocatalytic activity can be improved by creation of defects in the crystal lattice of 2D layered transition metal dichalcogenides (TMDCs) or ternary metal chalcogenides (TMCs) such as MoS2 or Cu2MoS4, respectively. However, the role of anion substitution in the enhancement of overall electrocatalytic activity in TMCs remains unproven. Here, we show the substitution of anion atom sulfur (S) with selenium (Se) in a new electrocatalyst Cu2WS4 for efficient hydrogen evolution reaction (HER) activity. The higher electrocatalytic activity of Cu2WS4 after anion atom substitution can be attributed to the creation of chemical strain in the lattice, which causes an increase of active sites for hydrogen adsorption and desorption. Experimentally, the anion substituted Cu2W(SySe1 − y)4 samples show superior electrocatalytic activities with a low onset potential of −0.320 V at 10 mA cm−2 for the HER, which is two-fold lower than that of the pristine Cu2WS4 (−0.650 V at 10 mA cm−2) sample. In addition, after 1000 cycles with continuous electrolysis in an acid electrolyte for 12 h, the anion substituted samples Cu2W(SySe1 − y)4 preserve their structure and robust catalytic activity perfectly. As a result, our work demonstrates a new approach for developments of real applications of TMCs in energy conversion.


 Please wait while we load your content...
Please wait while we load your content...