Enabling a highly reversible conversion reaction in a lithiated nano-SnO2 film coated with Al2O3 by atomic layer deposition
Abstract
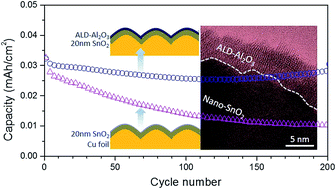
The huge decay in the reversible capacity and low initial coulombic efficiency (ICE) of a lithiated SnO2 film anode originate mainly from the limited reversibility of the conversion reaction. The inhibition of Sn coarsening is the key to promote the reversible conversion of Sn/Li2O to SnO2. Herein, we designed a double-layer electrode comprising a SnO2 layer (thickness of ∼20 nm) coated with an Al2O3 layer by atomic layer deposition as a conceptual model. The Al2O3 coating (∼5 nm) on the surface and the interior filling of Al2O3 helped to increase the boundary integrity of SnO2 nanocrystals, and acted as a barrier to prevent lithiation-induced Sn coarsening during cycling, ensuring a highly reversible conversion reaction in the SnO2/Al2O3 film electrode. Thus, this SnO2/Al2O3 electrode contributed superior electrochemical performance with a higher ICE (88.1%) and better reversible capacity retention (88.9% after 200 cycles) than the pure SnO2 anode (30.6%); this demonstrated that the surface modification of nanograins of conversion-type anode materials could be helpful for enhancing the reversibility and cyclability of the electrode and enabling a high stable reversible capacity.


 Please wait while we load your content...
Please wait while we load your content...