Towards enhanced sodium storage by investigation of the Li ion doping and rearrangement mechanism in Na3V2(PO4)3 for sodium ion batteries†
Abstract
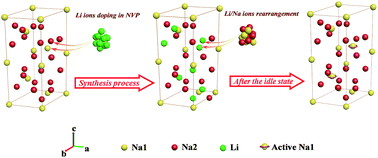
Metal ion doping is an effective way for improving the intrinsic properties of Na3V2(PO4)3. However, mechanistic exploration of metal ion doping in Na3V2(PO4)3, which is essential for the design and optimization of metal ion doping, is indistinct. To achieve an in-depth understanding of the mechanism of metal ion doping, Li ion doped Na3−xLixV2(PO4)3/C (x = 0, 0.01, 0.05, 0.1, 0.5, 0.7 and 1.0) compounds are synthesized. By utilizing DFT, NMR and Rietveld methods, the mechanism of Li/Na ion doping and rearrangement in the Na sites and Na ion insertion/extraction during electrochemical cycles are explored and identified. It is found that during the synthesis process, Li ions are inclined to enter mainly the Na2 sites in Na3V2(PO4)3 when the x value is low, but they will occupy the Na1 and Na2 sites simultaneously when x is high. Meanwhile, Li ions in the as-prepared Na3−xLixV2(PO4)3 compounds are reversibly replaced by Na ions during the idle state, due to the very small energy difference of the exchange between Li and Na ions. Furthermore, it is confirmed that there are more than two Na ions inserted/extracted during the charge–discharge process, resulting in the final extra specific capacity.


 Please wait while we load your content...
Please wait while we load your content...