Elastic Ag-anchored N-doped graphene/carbon foam for the selective electrochemical reduction of carbon dioxide to ethanol†
Abstract
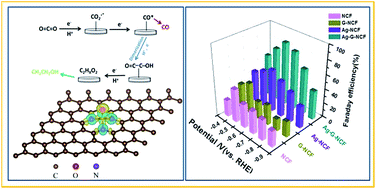
Electrochemical reduction of CO2 is considered to be an efficient strategy for converting CO2 emissions into valued-added carbon compounds. However, it often suffers from high overpotential, low product faradaic efficiency and poor selectivity for the desired products. Herein, a cost-effective method was designed to anchor Ag nanoparticles onto 3D graphene-wrapped nitrogen-doped carbon foam (Ag-G-NCF) by direct carbonization of melamine foam loaded with graphene oxide and silver salt. Directly acting as a high-efficiency electrode for CO2 electrochemical reduction, the Ag-G-NCF can efficiently and preferentially convert CO2 to ethanol with faradaic efficiencies (FEs) of 82.1–85.2% at −0.6 to −0.7 V (vs. RHE), overcoming the usual limitation of low FE and selectivity for C2 products. Density functional theory calculations confirmed that the pyridinic N species of the Ag-G-NCF catalyst exhibited a higher bonding ability toward CO* intermediates than other N species, and that then the Ag particles gradually converted the CO* to the OC–COH intermediate of ethanol. Its excellent performance in CO2 electroreduction can be attributed to a combination of the synergistic catalysis occurring between the pyridinic N present at high content and the Ag nanoparticles, the hierarchical macroporous structure, and the good conductivity.


 Please wait while we load your content...
Please wait while we load your content...