Nickel metal–organic framework implanted on graphene and incubated to be ultrasmall nickel phosphide nanocrystals acts as a highly efficient water splitting electrocatalyst†
Abstract
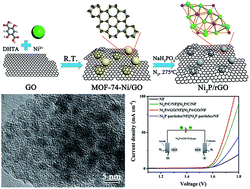
The development of low-cost, efficient, and stable electrocatalysts with bifunctional catalytic activity for overall water splitting is desirable but remains a great challenge. Here, a template-confinement strategy is presented with nickel metal–organic framework (MOF-74-Ni) implanted on graphene oxide and incubated by low temperature phosphorization to become ultrasmall nickel phosphide nanocrystals anchored on reduced graphene oxide (termed Ni2P/rGO). The size-controlled synthesis of ultrasmall metal-based catalysts is of vital economic interest and scientific importance for chemical conversion technologies. The Ni2P/rGO guarantees large active surface area and perfect dispersity of the active sites with ultrasmall particle sizes (average about 2.6 nm), which can serve as a highly efficient electrocatalyst for overall water splitting. In 1.0 M KOH, the Ni2P/rGO exhibited remarkable electrocatalytic performance for both HER and OER, affording a current density of 10 mA cm−2 at overpotentials of 142 mV for HER and 260 mV for OER with small Tafel slope. Furthermore, an electrolyzer employed with Ni2P/rGO as a bifunctional catalyst in both the cathode and anode in 1.0 M KOH generated 10 mA cm−2 at a voltage of 1.61 V with excellent stability, comparable to the integrated Pt/C and RuO2 counterparts, which is among the best performances of transition metal phosphides (TMPs).


 Please wait while we load your content...
Please wait while we load your content...