La2CoO4: a new intercalation based cathode material for fluoride ion batteries with improved cycling stability
Abstract
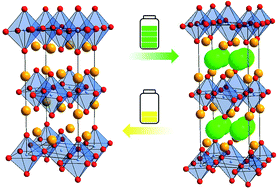
In this study, we report on the electrochemical cycling behavior of La2CoO4 (against a composite of Pb/PbF2 as the anode material) for use as an intercalation-based cathode material for fluoride ion batteries (FIBs). The material can intercalate approximately 1.2 fluoride ions per formula under the formation of La2CoO4F1.2 resulting in a strong increase of the cell volume, confirmed by means of ex situ X-ray diffraction studies at various charging capacities and additional complementary chemical fluorination experiments. Furthermore, by only regulating the cut-off capacity we were able to remarkably avoid unwanted side reactions which were previously assumed to be a major problem for achieving high cycling numbers of LaSrMnO4. In this respect, electrochemical impedance spectroscopy was used to determine an initial critical specific charge capacity of ∼65 mA h g−1. By carefully designing the charging process, a discharge capacity as high as 32 mA h g−1 could be obtained, which is the highest capacity for an intercalation based cathode material reported so far, with a capacity retention of ∼25% of the initial discharge capacity after 50 cycles. In addition, we discuss why avoiding the cross-reactivity of the carbon additive is more difficult for fluoride ion batteries than for lithium ion batteries, showing that this is an important challenge for the successful implementation of high voltage cathode materials.


 Please wait while we load your content...
Please wait while we load your content...