Improved interfacial H2O supply by surface hydroxyl groups for enhanced alkaline hydrogen evolution†
Abstract
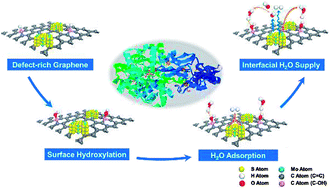
Robust hydrogen evolution reaction (HER) in alkaline solution using a cost-effective catalyst is still a critical challenge for industrial H2O electrolysis. The dramatic depletion of interfacial H2O and hindered OH− diffusion bring about a harsh interfacial environment and thereby lead to sluggish hydrogen evolution. Here we develop heterogeneous catalysts based on defect-rich three-dimensional graphene (M/3D graphene) and achieve improved HER kinetics via surface hydroxyl group modification on the graphene surface. Our theoretical studies suggest that the surface hydroxyl groups derived from the hydroxylation process effectively attract multi-overlayer H2O clusters without any thermodynamic barrier and form a reservoir to continuously supply H2O for catalytic sites. Electrochemical characterizations indicate that the HER kinetics per active electrochemical surface area have been greatly improved after crafting abundant hydroxyl groups on the 3D graphene framework, which can be attributed to sufficient proton donors and an ameliorative interfacial pH environment. Benefiting from the surface hydroxyl group modification, the MoS2/3D graphene structure facilitates the HER at a low onset potential of 60 mV in alkaline medium and exhibits excellent durability after 3000 cycles. This surface modification strategy reveals the importance of interfacial H2O supply in HER kinetics and provides general guidance on designing highly efficient catalysts in alkaline medium.


 Please wait while we load your content...
Please wait while we load your content...