Are we underrating rare earths as an electrocatalyst? The effect of their substitution in palladium nanoparticles enhances the activity towards ethanol oxidation reaction†
Abstract
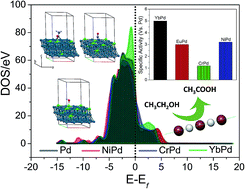
Since the advent of catalysis, transition metal-based materials have continually been exploited as efficient electrocatalysts, whereas rare-earths (REs) have been neglected due to their misleading name, i.e. rare-earth, and cost. In fact, most REs are abundant and less expensive than the most explored transition metals. In view of this, we attempted to study the chemical effects of small amounts of RE (10%) substitution in the Pd lattice (REPd) for comparison with transition metal-substituted Pd (TMPd) towards the ethanol oxidation reaction (EOR). The electrochemical activities of REPd (RE = Eu and Yb) towards EOR were found to show many fold increases in both specific and mass activities as compared to those of TMPd (TM = Cr and Ni) and commercial Pd/C. Theoretical investigations assisted by DFT calculations support the experimental observations with a perfect synergy between the adsorption energies of –OH and –COCH3 to the catalyst surface, which provides unprecedented catalytic activity for REPd as compared to the case of other catalysts. The promotional effects of RE were well exploited in enhancing the activity and stability of Pd towards EOR, as observed by electrochemical studies, X-ray absorption near edge spectroscopy and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...