Two-dimensional VS2 monolayers as potential anode materials for lithium-ion batteries and beyond: first-principles calculations†
Abstract
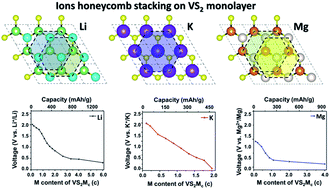
First-principles calculations based on density functional theory were carried out to investigate the electrochemical performance of monolayer VS2 for Li-, K-, Mg- and Al-ion batteries. A VS2 monolayer shows differential storage ability for various cations, able to adsorb three layers of Li, two layers of Mg, one layer of K, and 1/9 layer of Al on both sides of the monolayer, producing theoretical capacities of 1397, 1863, 466, and 78 mA h g−1 for Li, Mg, K, and Al, respectively. The average working voltages of VS2 monolayers for Li+, K+ and Mg2+ are close to those of metallic Li, K, and Mg, suggesting that they can be used as anode materials in these rechargeable batteries. The adsorbed cations form a honeycomb-stacking lattice on VS2 monolayers, similar to the plating process of Li, K, and Mg metal anodes. More interestingly, the honeycomb Li lattice is different from the body-centered cubic lattice of a Li metal anode, which provides very small diffusion barriers, resulting in the high rate capability of VS2 monolayer in Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...