Powerful synergy: efficient Pt–Au–Si nanocomposites as state-of-the-art catalysts for electrochemical hydrogen evolution†
Abstract
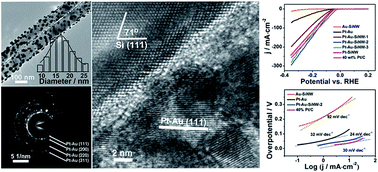
The hydrogen evolution reaction (HER) is a fundamental electrochemical reaction to produce hydrogen gas from the electrolysis of water, and has been regarded as an attractive solution to many energy challenges. In the past few decades, platinum has been widely demonstrated to split water into hydrogen gas at high reaction rates and low overpotentials. Nevertheless, decreasing the loading of Pt in the designed electrocatalysts is very significant. However, with low Pt loading, it is challenging to maintain excellent catalytic performance. Here we report a Pt–Au–Si ternary nanocomposite as a highly active catalyst for the HER. The optimal composition of Pt–Au–Si was determined to be 7.2 : 31.3 : 61.5 (Pt : Au : Si, mass ratio) with a Tafel slope of only 24 mV dec−1. To be specific, the mass activity of the Pt–Au–SiNW-2 catalyst was 6.5 fold that of a 40 wt% Pt/C catalyst at an overpotential of 60 mV. More importantly, the cathodic current density even surpassed 40 wt% Pt/C when the overpotential was larger than 0.17 V (the corresponding current density was 108 mA cm−2). The outstanding catalytic performance might be derived from the synergy of the respective properties of the three components: the fast hydrogen adsorption rate on Pt, the quick migration of the adsorbed hydrogen atoms on Au and the rapid hydrogen evolution rate on Si. This research opens up a novel strategy to prepare highly efficient catalysts for electrochemical hydrogen evolution reactions.


 Please wait while we load your content...
Please wait while we load your content...