Cu2ZnSnS4 and Cu2ZnSn(S1−xSex)4 nanocrystals: room-temperature synthesis and efficient photoelectrochemical water splitting†
Abstract
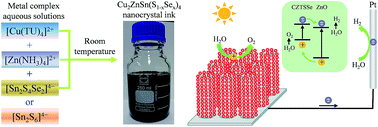
Green synthesis of Cu2ZnSnS4 (CZTS) and Cu2ZnSn(S1−xSex)4 (CZTSSe) nanocrystals is highly desirable for low-cost and high-efficiency solar energy conversion devices. In this work, scalable synthesis of multinary CZTS and CZTSSe nanocrystals at room temperature has been achieved by a simple metal complex solution mixing (Metcomix) process. In the Metcomix process, CZTS or CZTSSe nanocrystals are formed by simply mixing aqueous solutions of copper thiourea complex ([Cu(TU)4]2+), zinc ammonium complex ([Zn(NH3)4]2+) and tin chalcogen complex ([Sn2S6]4−) or tin double chalcogen complex ([Sn2S4Se2]4−) at room temperature. The Metcomix process features low-energy-consuming, low-cost, environmentally friendly, high-purity, and scalable-production. The CZTS and CZTSSe nanocrystals have a small size of 4–10 nm and exhibit remarkable room-temperature photoluminescence and optical absorption properties. The CZTS and CZTSSe nanocrystals are also deposited onto ZnO nanorod arrays and demonstrated as efficient photoanodes for photoelectrochemical water splitting. The ZnO/CZTSSe photoanode exhibits a photocurrent density of 9.06 mA cm−2 at 1.23 V (vs. the NHE) and an optimal applied bias photon-to-current efficiency (ABPE) of ∼3.43% at a bias of 0.60 V. The present work demonstrates a new approach for synthesizing eco-friendly multinary chalcogenide nanocrystals at room temperature and their promising applications in solar energy conversion devices.


 Please wait while we load your content...
Please wait while we load your content...