MnCo2O4 decorated Magnéli phase titanium oxide as a carbon-free cathode for Li–O2 batteries†
Abstract
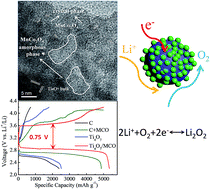
Advanced cathode catalysts are crucial to the promotion of aprotic Li–O2 batteries for practical applications. Carbon is usually used as a cathode catalyst, but it reacts with the discharge products (Li2O2, LiO2) to form an insulating layer of lithium carbonate and prevents further reaction. To resolve this issue, the development of non-carbon cathode catalysts is of great demand. Herein, for the first time, we designed and fabricated a MnCo2O4 (MCO) spinel oxide decorated Magnéli phase Ti4O7 as a carbon-free cathode for Li–O2 batteries. The sub-stoichiometric Ti4O7 oxide serves as an electronic conductive network. The MCO spinel oxide along with the synergistic effect between Ti4O7 and MCO facilitate the kinetics of both oxygen reduction and decomposition of Li2O2. Furthermore, uniform anchoring of MCO nanoparticles on Ti4O7 surface provides a stable lithium peroxide–cathode interface during the discharge/charge process. The resulting Ti4O7/MCO hybrid proves to be a highly effective cathode catalyst. The discharge/charge voltage gap of the Ti4O7/MCO hybrid is about 0.75 V, which is significantly lower than that of pure carbon, C + MCO and pristine Ti4O7 cathode. A high specific capacity (5400 mA h g−1 at 100 mA g−1) and excellent cycling performance (100 cycles at a capacity of 500 mA h g−1 under 200 mA g−1) were obtained for this hybrid. The high catalytic activity and durability renders the Ti4O7/MCO hybrid a highly promising carbon-free cathode for Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...