Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg(ii)†
Abstract
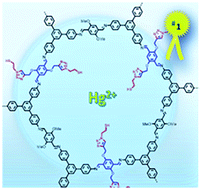
An imine-linked covalent organic framework ([HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.5-TPB-DMTP-COF), endowed with reactive ethynyl groups on the walls of one-dimensional pores, has been used as a platform for pore-wall surface engineering with triazole and thiol groups to yield TPB-DMTP-COF-SH, which is suitable to interact very efficiently with mercury ions. The evaluation of the carefully designed TPB-DMTP-COF-SH is addressed as an effective and selective system for mercury sorption. The obtained results reveal an extraordinary capacity and a great efficiency of the polymeric material with a very high distribution coefficient value Kd = 3.23 × 109. Thus, the level of mercury of a highly concentrated aqueous solution, 10 mg L−1 of Hg(II), is dramatically decreased, below the limits of what is considered to be drinking water, upon treatment with TPB-DMTP-COF-SH for a few minutes. The TPB-DMTP-COF-SH retention value of Hg(II) from water is 99.98% within 2 minutes and its record uptake capacity is 4395 mg g−1 which represents the highest value reported so far. Besides TPB-DMTP-COF-SH captures other extremely toxic heavy metal ions such as Sn(II) and Pb(II) being quite selective versus Cd(II) or As(III). These results suggest that TPB-DMTP-COF-SH constitutes a realistic alternative for the remediation of contaminated spaces from an environmental perspective.
C]0.5-TPB-DMTP-COF), endowed with reactive ethynyl groups on the walls of one-dimensional pores, has been used as a platform for pore-wall surface engineering with triazole and thiol groups to yield TPB-DMTP-COF-SH, which is suitable to interact very efficiently with mercury ions. The evaluation of the carefully designed TPB-DMTP-COF-SH is addressed as an effective and selective system for mercury sorption. The obtained results reveal an extraordinary capacity and a great efficiency of the polymeric material with a very high distribution coefficient value Kd = 3.23 × 109. Thus, the level of mercury of a highly concentrated aqueous solution, 10 mg L−1 of Hg(II), is dramatically decreased, below the limits of what is considered to be drinking water, upon treatment with TPB-DMTP-COF-SH for a few minutes. The TPB-DMTP-COF-SH retention value of Hg(II) from water is 99.98% within 2 minutes and its record uptake capacity is 4395 mg g−1 which represents the highest value reported so far. Besides TPB-DMTP-COF-SH captures other extremely toxic heavy metal ions such as Sn(II) and Pb(II) being quite selective versus Cd(II) or As(III). These results suggest that TPB-DMTP-COF-SH constitutes a realistic alternative for the remediation of contaminated spaces from an environmental perspective.


 Please wait while we load your content...
Please wait while we load your content...