Facile and one-step synthesis of a free-standing 3D MoS2–rGO/Mo binder-free electrode for efficient hydrogen evolution reaction†
Abstract
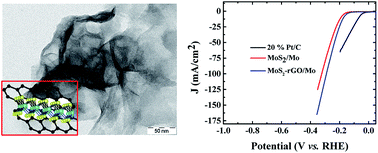
A free-standing 3D MoS2/Mo electrode was prepared via a facile, one-step hydrothermal method wherein the Mo foil itself acted as the Mo source and thio-urea as the S source. When directly used as a hydrogen evolution reaction (HER) electrode, the as-prepared MoS2/Mo exhibited a low onset potential of 120 mV, requiring an overpotential of only 334 mV to reach a current density of 100 mA cm−2 while maintaining its catalytic activity for over 20 h. The activation energy of the reaction was determined to be ∼120 meV by temperature-dependent HER studies. In addition, we have also shown that MoS2 structures can be repetitively grown on Mo foil for subsequent HER application by etching MoS2 with H2O2. The HER performance was enhanced further by incorporating reduced graphene oxide (rGO) into the 3D MoS2/Mo electrode, which facilitated better electron transfer through rGO. The onset potential of the MoS2–rGO/Mo was 100 mV (which is 20 mV lower than that of the MoS2/Mo electrode) and it required 291 mV to achieve a current density of 100 mA cm−2. The catalysts were also found to be highly durable.


 Please wait while we load your content...
Please wait while we load your content...