A conversion-based highly energy dense Cu2+ intercalated Bi-birnessite/Zn alkaline battery
Abstract
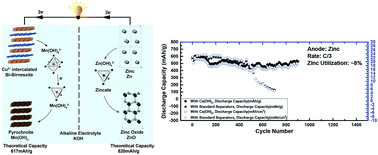
Manganese dioxide (MnO2)–zinc (Zn) batteries are cheap and environmentally benign and have sufficient theoretical energy density to be used as an energy storage device for the grid; however, they have been relegated to primary systems, where the complete energy is delivered in a single discharge, due to the irreversibility of their active materials. Until recently, rechargeable MnO2–Zn batteries have only been able to cycle ∼10% of MnO2's theoretical 2-electron capacity (617 mA h g−1), thus delivering significantly reduced energy density. In a recent paper from our group, we reversibly accessed the full theoretical 2-electron capacity of MnO2 for >6000 cycles by using a layered polymorph of MnO2 mixed with bismuth oxide (Bi2O3) called Bi-birnessite (Bi–δ-MnO2) intercalated with Cu2+ ions. This discovery highlighted the possibility of achieving very high energy densities from inexpensive aqueous batteries; however, a full-cell demonstration with Zn as the anode was not studied. Here we report for the first time the effect of Zn anodes on the cycle life and energy density of a full cell, where we observe that 15% depth-of-discharge (DOD) of the Zn's theoretical capacity (820 mA h g−1) creates a cell energy density of ∼160 W h L−1; however, this causes a drastic shape change and formation of irreversible zinc oxide (ZnO) at the anode, which ultimately causes cell failure after ∼100 cycles. A drop in energy density is also observed as a result of the interaction of dissolved Zn ions with the cathode, which forms a resistive Zn-birnessite compound in the early cycles, and then forms a highly resistive haeterolite (ZnMn2O4) in the later cycles, and ultimately causes cathode failure. A possible solution using a calcium hydroxide layer as a separator is presented, where the layer blocks the interaction of zinc ions through a complexing mechanism to obtain >900 cycles with >80% retention of MnO2 DOD.


 Please wait while we load your content...
Please wait while we load your content...