A multi-functional gum arabic binder for NiFe2O4 nanotube anodes enabling excellent Li/Na-ion storage performance†
Abstract
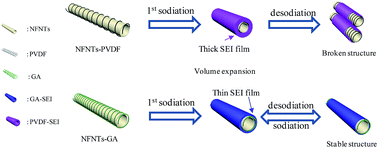
Electrode pulverization, low electrochemical reaction kinetics and an unstable SEI layer have prevented the application of transition metal oxides with a conversion-type mechanism. Here, we describe gum arabic (GA) as a green and multi-functional binder for the fabrication of a NiFe2O4 nanotube (NFNT) electrode enabling predominant application in LIBs and NIBs. Firstly, it's revealed that the NFNTs–GA electrode possesses better mechanical properties of a higher friction coefficient, better elastic resilience and higher reduced modulus and hardness compared with a NFNTs–PVDF electrode. Secondly, the NFNTs–GA electrode can restrain the side reactions between the electrode and electrolyte, leading to the formation of a remarkably stable and thin SEI layer during discharge and charge processes. Thirdly, it is demonstrated by KPFM that the NFNTs–GA electrode possesses improved surface electrical properties and lower energy for the escape of electrons. Consequently, the NFNTs–GA electrode demonstrates much improved rate capability, cycling stability and columbic efficiency when used as an anode material for LIBs. It displays a stable capacity of 770 mA h g−1 which can be retained after 500 cycles at 0.5 A g−1. More importantly, the NFNTs–GA electrode exhibits a high initial coulombic efficiency of 73% (only 48% for the NFNTs–PVDF electrode) and enhanced electrochemical reaction kinetics with significantly improved oxidation and reduction peaks in the application of NIBs.


 Please wait while we load your content...
Please wait while we load your content...