Poly(vinylidene fluoride)-based hybrid gel polymer electrolytes for additive-free lithium sulfur batteries†
Abstract
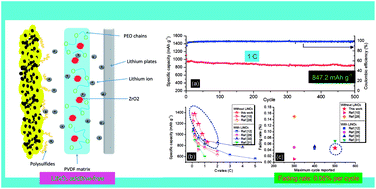
The permeation of dissolved lithium polysulfides across the porous polyolefin-based commercial separator is a major hindrance for using lithium sulfur batteries (LSBs). In this work, the poly(vinylidene fluoride) (PVDF)-based gel polymer electrolyte (GPE) with a compact morphology to block polysulfide penetration is prepared using a simple solution-casting method, and the strategy of incorporating poly(ethylene oxide) and nano zirconium dioxide is applied to guarantee electrolyte uptake and Li+ mobility. Superior to the commercial separator with liquid electrolyte, the LSB assembled with additive-free GPE exhibits a high initial capacity of 1429 mA h g−1, coulombic efficiency of 96% at 0.2C and improved rate performance. After 500 cycles at 1C, the LSB could still deliver a capacity of 847.2 mA h g−1, with a low fading rate of 0.05%. The LSB with high sulfur loading (5.2 mg cm−2) could attain a high areal capacity of 4.6 mA h cm−2. Results of scanning electron microscopy suggest that such a hybrid GPE could effectively protect the lithium anode from polysulfide corrosion. Therefore, this novel membrane of hybrid PVDF-based GPE provides a simple and effective method to establish high-performance LSBs.


 Please wait while we load your content...
Please wait while we load your content...