Iron oxyfluorides as lithium-free cathode materials for solid-state Li metal batteries†
Abstract
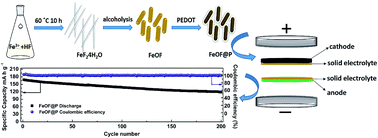
High energy density storage systems can be achieved by utilizing a lithium metal anode in solid-state Li secondary batteries. Herein, we report for the first time the use of a lithium-free cathode coupled with lithium metal for solid-state Li metal batteries. This lithium-free cathode composed of iron oxyfluoride (FeOF) nanorods can not only reduce the consumption of lithium resources, but also provide a good electrochemical performance. After being encapsulated by a highly conductive poly(3,4-ethylenedioxythiophene) (PEDOT) nanolayer, the coated FeOF nanorods exhibit a superior cycling stability (ca. 75% capacity retention after 200 cycles at 100 mA g−1) at 60 °C in a PEA solid electrolyte. This performance demonstrates the great potential of lithium-free cathodes for application in solid-state Li metal batteries.


 Please wait while we load your content...
Please wait while we load your content...