Fabrication of an advanced asymmetric supercapacitor based on a microcubical PB@MnO2 hybrid and PANI/GNP composite with excellent electrochemical behaviour†
Abstract
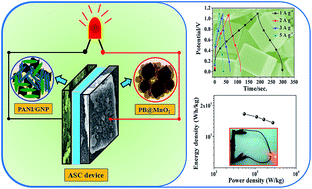
Metal Organic Framework (MOF) based supercapacitors are one of the best energy storage devices for future portable electronics. In this study, we report in detail the design and low-cost fabrication of an advanced asymmetric supercapacitor (ASC) which was assembled with a Prussian blue (PB)/MnO2 (PB@MnO2) hybrid as the positive electrode and a polyaniline (PANI)/graphene nanoplatelet (GNP) (PG) composite as the negative electrode with aq. KNO3 electrolyte. Both the electrodes were made by the coating of the respective electrode materials on conducting stainless steel (SS) fabric. The MOF based positive electrode material, i.e., the PB@MnO2 hybrid was synthesized via reducing agent assisted chemical bath deposition of MnO2 nanolayers on faradaic PB microcubes and showed an appreciable specific capacitance (Csp) of 608 F g−1 at 1 A g−1 current density in the three-electrode measurement and the assembled PB@MnO2//PG ASC device manifests favourable Csp of 98 F g−1 at 1 A g−1. Moreover, this ASC device exhibits significant energy density of 16.5 W h Kg−1 at the power density of 550 W Kg−1 along with notable long-term cycling stability (retention of 93% capacitance even after 4000 cycles of charging and discharging). Thus, the obtained results reflect the great potential of the ASC device for exploring state-of-art futuristic applications as an advanced energy storage system.


 Please wait while we load your content...
Please wait while we load your content...