Electrochemical determination of the gallium-nitride photocorrosion potential in acidic media
Abstract
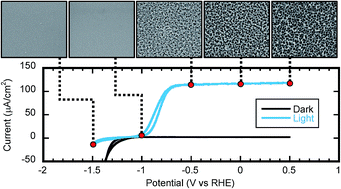
Gallium nitride-based semiconductors have demonstrated their usefulness as efficient light absorbers with fast kinetics for photoelectrochemical water splitting, but they still need to demonstrate long lifetime stability in aqueous solutions. It is therefore necessary to understand how corrosion reaction potentials relate to the water splitting reactions, and to the conduction and valence bands of the semiconductor light absorber/reactor. In this study the previously established acidic media photocorrosion reaction of GaN to Ga3+ and 1/2N2 is electrochemically investigated. By using Mott–Schottky analysis the photocorrosion potential is found to be −0.66 ± 0.07 V vs. RHE (pH 1) relating it to Ga surface states acting as hole traps. The photocorrosion potential found by Mott–Schottky analysis is found to also relate to the Fermi level of GaN before and after etching as determined by XPS. SEM imaging is additionally used to confirm the photocorrosion potential, and shows that introducing hole scavengers to the electrolyte can limit the rate of photocorrosion.


 Please wait while we load your content...
Please wait while we load your content...