P2-type transition metal oxides for high performance Na-ion battery cathodes†
Abstract
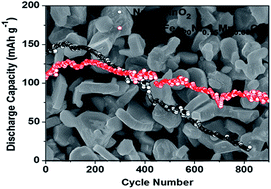
The particle pulverization induced by volume change and the disproportionation reaction of Mn3+ to Mn4+ and electrolyte-soluble Mn2+ are two major challenges for Na0.67MnO2 cathodes in Na-ion batteries. Herein, Ni and/or Fe doped Na0.67MnO2 was synthesized to suppress the particle pulverization and disproportionation reaction. The replacement of 33% Mn ions by Ni in Na0.67MnO2 can effectively reduce the particle pulverization and disproportionation of Mn3+, resulting in improved cycling stability at the cost of reduced capacity. To develop a high capacity and long cycle life cathode material, Ni in Na0.67Ni0.33Mn0.67O2 is further partially substituted by Fe to generate Na0.67Fe0.20Ni0.15Mn0.65O2, which retains ∼70% of its initial capacity after 900 cycles, corresponding to a very low capacity decay rate of 0.033% per cycle. To the best of our knowledge, the Na0.67Fe0.20Ni0.15Mn0.65O2 synthesized by ultrasonic spray pyrolysis (USP) represents one of the best cathode materials for Na-ion batteries to date. In addition, a thin layer (5 nm) of Al2O3 is deposited on the Na0.67MnO2 electrode by atomic layer deposition (ALD) to further reduce the dissolution of Mn ions and accommodate the volume change, which further extend the cycling stability of Na0.67MnO2 electrodes.


 Please wait while we load your content...
Please wait while we load your content...