Hydrogenation of silicene on Ag(111) and formation of half-silicane
Abstract
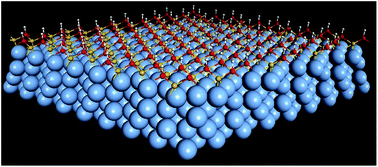
Functionalization of silicene on metal substrates represents a key step for applications of silicene in future nanoelectronic devices. We find similarities in the electronic bands of 2√3 × 2√3R30° silicene and other silicene superstructures such as √13 × √13R13.9° and 4 × 4, that is, silicene π electrons lose their two-dimensional (2D) honeycomb character while σ states turn out to be less interacting and moderately affected. Half-silicane with a prefect 1 × 1 periodicity is formed on Ag(111), with one silicon sublattice accommodating the hydrogen atoms. Hydrogenation rearranges the out-of-plane buckling of silicene on Ag(111), and the energy of silicon σ states is raised toward the Fermi level (EF) due to the change of the buckling configuration. The formation of half-silicane results in a new type of silicene monolayer with a large buckling height. Dehydrogenation occurs at moderate temperatures, demonstrating that such uniformly ordered and reversible hydrogenation may be useful for tuning the properties of silicene and for controllable hydrogen storage.


 Please wait while we load your content...
Please wait while we load your content...