All-solid-state disordered LiTiS2 pseudocapacitor†
Abstract
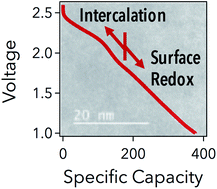
Pseudocapacitive materials offer an opportunity to bridge the energy storage gap between supercapacitor and battery technologies. Herein is chronicled the first report of pseudocapacitance in a system devoid of liquid electrolytes, using the cathode material LiTiS2. It is demonstrated that due to extreme crystallite reduction to less than 3 nm, additional charge storage is derived by reducing surface Ti3+ to Ti2+ at higher voltages and more reversibly than traditionally shown. Due to facile diffusion pathways in 3-fold coordinated lithium along the TiS2 surfaces, disordered LiTiS2 can be used as a singular cathode without conductive additives. The result is a system exhibiting nearly 300 mA h g−1 at a rate of C/2 for 1000 cycles. Whereas active materials in liquid cells typically have size limitations before irreversibilities appear, the high pseudocapacitance demonstrated in this report indicates that active materials used in the solid-state could benefit from size reduction. Hopefully, a new avenue of research stems from this work to investigate mixed conductor nano-domains for solid-state battery/capacitor hybrids. The prospect of a solid-state pseudocapacitor unlocks a series of new applications that offer long shelf life, high temperature capabilities, and enhanced safety.


 Please wait while we load your content...
Please wait while we load your content...