Influence of Cu2+ doping concentration on the catalytic activity of CuxCo3−xO4 for rechargeable Li–O2 batteries†
Abstract
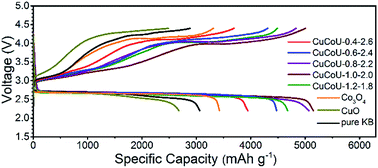
The electrochemical performance of rechargeable lithium–oxygen (Li–O2) batteries relies on the catalysts used in the cathode to a great extent. Herein, a series of CuxCo3−xO4 nanorods with different Cu2+ concentrations was prepared by a convenient hydrothermal method. The corresponding structures and electrochemistries were further characterized in order to reveal the composition effect of catalyst on catalytic activity. Experimental characterization and theoretical calculations indicate that more Cu2+ occupying Co2+ positions and dispersing on the catalyst surface has an enhanced catalytic activity in terms of higher capacity, lower overpotential and better reversibility. This implies that suitable charge transfer from Li2O2 to the catalyst plays an important role in improving the electrochemical performance of Li–O2 batteries. The present study is helpful for designing a highly active catalyst by tuning the composition of the catalyst surface.


 Please wait while we load your content...
Please wait while we load your content...