Synthesis of cryptomelane type α-MnO2 (KxMn8O16) cathode materials with tunable K+ content: the role of tunnel cation concentration on electrochemistry†
Abstract
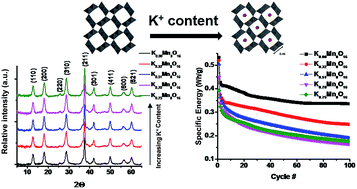
The role of tunnel cations in the electrochemistry of α-MnO2 materials has been long discussed and demands investigation as the electrochemistry of α-MnO2 materials is strongly dependent on the material specific properties (i.e. morphology, surface area, crystallite size, and chemical composition). Here, we systematically synthesized a series of α-MnO2 samples with differing K+ content but similar physicochemical and morphological properties allowing direct investigation of the role of tunnel cation (K+) on the lithium ion electrochemistry of α-MnO2 cathodes. The nanofibrous α-MnO2 materials have a chemical composition of KxMn8O16·yH2O, where 0 ≤ x ≤ 0.75 and 0.53 ≤ y ≤ 0.81. The α-MnO2 materials have similar morphology, crystallite size (17–19 nm), surface area (66–76 m2 g−1), and tunnel water content (0.53–0.81). The electrochemistry of the α-MnO2 materials was evaluated using cyclic voltammetry, galvanostatic cycling, and galvanostatic intermittent titration type tests. The α-MnO2 materials with 0 to 0.32 K+ content showed discharge curves with higher voltage, higher specific energies, and improved capacity retention compared to the 0.75 K+ containing α-MnO2 material. Fewer structural distortions were observed in lithiated samples with lower K+ content through modelling of X-ray absorption spectroscopy data indicating improved structural stability of those samples which positively impacted the electrochemistry.


 Please wait while we load your content...
Please wait while we load your content...