Enhancement in kinetics of the oxygen reduction on a silver catalyst by introduction of interlaces and defect-rich facets†
Abstract
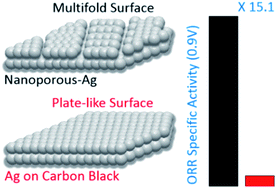
Controlling the nanostructure of a metallic catalyst has become an efficient strategy to tune and to optimize its catalytic properties for electrochemical reactions. Reported herein is a unique nanoporous silver (np-Ag) structure, a multigrain, whose surface is predominantly enclosed by interlaced facets and thus defect-enriched surfaces. The synthesis is accomplished by a simple and efficient method involving hydrothermal treatment of a composite containing Ag+ ions and reduced-polyoxometalate nanoclusters. Experimental results show that the novel np-Ag catalyst can electrocatalyze the oxygen reduction reaction (ORR) with mass (6.90 mA mg−1Ag) and specific (0.068 mA cm−2Ag) activities are enhanced 16.2 and 15.1 times relative to those of the Ag/C catalyst (0.425 mA mg−1Ag and 0.0045 mA cm−2Ag, respectively). The catalysts' excellent catalytic performance originates from the increased availability of surface active defects and the features of the porous structure. It is anticipated that this work will offer a new approach to surface-controlled synthesis and ORR catalysts design.


 Please wait while we load your content...
Please wait while we load your content...