Investigation of high oxygen reduction reaction catalytic performance on Mn-based mullite SmMn2O5†
Abstract
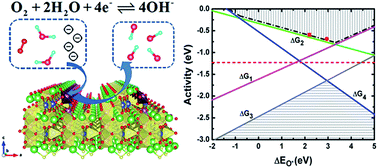
An alternative material SmMn2O5 mullite with regard to Pt/C is proposed to catalyze the oxygen reduction reaction (ORR) by combining density functional theory (DFT) calculations and experimental validations. Theoretical calculations are performed to investigate the bulk phase diagram, as well as the stability and electrocatalytic activity of the ORR under alkaline conditions for SmMn2O5 (001) surfaces, which are passivated by nitrogen atoms to avoid any spurious interference. The adsorptions of relevant ORR species (O*, OH*, OOH* and OO*) tend to compensate the coordination of manganese atoms to form Mn-centered octahedral or pyramidal crystal fields, and the corresponding binding energies fulfill a linear relationship. An oxygen molecule prefers to be reduced to OH−via a four-electron pathway and this prediction is verified by electrochemical measurements on the as-prepared SmMn2O5 catalyst with a nanorod structure. Volcano curves are obtained to describe the trends in theoretical ORR activity as a function of a single parameter, i.e. the oxygen binding energy. An overpotential of 0.43 V is obtained at the O* binding energy around 3.4 eV, which is close to the experimental observation (0.413 V) in this work. SmMn2O5 mullite exhibits favorable ORR activity and superior stability with only ∼5% decay in activity over 20 000 s of chronoamperometric operation in contrast to ∼15% decrease of Pt/C, making it a promising candidate for a cathode catalyst.


 Please wait while we load your content...
Please wait while we load your content...