Design of ultralong single-crystal nanowire-based bifunctional electrodes for efficient oxygen and hydrogen evolution in a mild alkaline electrolyte†
Abstract
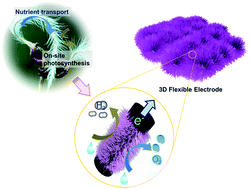
Rational design of bifunctional electrodes that efficiently pair oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) in a single system is critical for electrocatalytic pure oxygen and hydrogen generation and overall water splitting. Herein, we report a novel biomimetic architectural design and development of 3D flexible electrodes with high electrocatalytic activity and durability in a mild alkaline system. Benefiting from the ultralong single-crystal Co-doped ZnO nanowires densely and in situ grown on highly conductive carbon fabric, the novel material and unique composite electrode architecture favor the direct electrochemical water splitting process over the conventionally fabricated electrodes by providing abundant active sites, efficient electron transfer pathways, and sufficient gas exchange channels. A low overpotential of 360 and 275 mV is required to reach a current density of 10 mA cm−2 for OER and HER, respectively. When these thick electrodes are used as electrocatalysts for both anode and cathode in an overall water electrolyzer, a low cell voltage of 1.90 V and 2.06 V is required to reach a current density of 4.0 and 10.0 mA cm−2, respectively, with an extremely long durability. Such a single-cell prototype exhibiting superior performance to noble metal-based precious catalyst counterparts holds great promise in future practical applications for pure hydrogen generation.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...