Acetic acid-induced preparation of anatase TiO2 mesocrystals at low temperature for enhanced Li-ion storage†
Abstract
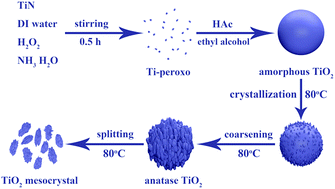
Titanium dioxide (TiO2) mesocrystals consist of a large number of subunits possessing distinct characteristics of large surface area, high crystallinity and large density and are considered as promising potential materials for energy storage and conversion, sensing, and photocatalysis. Therefore, it is significant to develop a robust and universal method to synthesize and precisely tune the TiO2 mesocrystals at low temperatures. However, this is still a big challenge and has not been largely achieved. In this context, we develop a robust method of synthesizing TiO2 mesocrystals by a facile hydrolysis and evolution process induced by acetic acid (HAc) solution at 80 °C using TiN as a starting material. The size and crystallinity of the TiO2 mesocrystals can be easily tuned by adjusting the HAc content and reaction time. The HAc governs the transformation of TiO2 from the amorphous state to the anatase phase at a low temperature (80 °C). The average size of the TiO2 mesocrystals can be tuned from ∼100 nm to 20 nm by changing the HAc content. TiO2 mesocrystals with a spindle-like morphology were formed and the tiny anatase TiO2 grains were grown along the same orientation [001] plane, which exhibits excellent electrochemical performance for lithium-ion storage. The specific capacity at 1C is 180 mA h g−1 and the retention is 92.9% after 100 cycles. Thereby, this study presents a universal method of crafting anatase TiO2 mesocrystals at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...