Spatial separation of photogenerated electron–hole pairs in solution-grown ZnO tandem n–p core–shell nanowire arrays toward highly sensitive photoelectrochemical detection of hydrogen peroxide†
Abstract
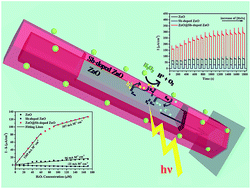
A facile solution process based on polyethylene glycol (PEG)-assisted secondary growth has been demonstrated for the epitaxial growth of a Sb-doped ZnO thin layer on ZnO nanowire arrays. The resultant ZnO@Sb-doped ZnO core–shell nanowires present a perfect single-crystal feature whereas structural deformation commonly existing in Sb-doped ZnO is greatly suppressed due to the confined thickness and the effect of PEG. Mott–Schottky analysis indicates that the formation of tandem n–p homojunctions at the coaxial interface is derived from the n-type conduction of the primary ZnO and the p-type conduction of the Sb-doped ZnO. By implementation of the core–shell nanowire arrays as electrodes for photoelectrochemical detection of the hole scavenger hydrogen peroxide (H2O2), a significantly enhanced photocurrent response toward different concentrations of H2O2 is obtained. The high sensitivity is ascribed to the efficient spatial separation of photogenerated electron–hole pairs facilitated by the built-in electric field in the n–p homojunction region, the lattice matching homojunction interface and the confined shell thickness for hole transport.


 Please wait while we load your content...
Please wait while we load your content...