The intrinsic properties of FA(1−x)MAxPbI3 perovskite single crystals†
Abstract
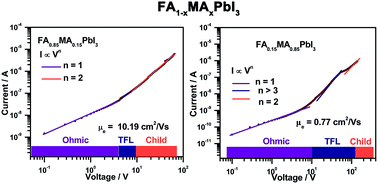
Organic–inorganic hybrid perovskites with mixed organic cations and/or halides have attracted increasing attention due to their superior optoelectronic properties, which are tailorable for different applications. To obtain a deeper understanding of materials properties, single crystals are regarded as the best platform among various building blocks for fundamental study. Here, we synthesized a series of perovskite single crystals with mixed organic cations (APbI3, A = CH3NH3+, MA+; or CH(NH2)2+, FA+) along the compositional space, and conducted a systematic investigation to correlate the carrier behavior with the organic cations. The single crystals were synthesized via inverse temperature crystallization assisted by hydroiodic acid, where the quality of the crystals could be judiciously controlled by the thermodynamic process. It is found that the substitution of 15% MA+ in FAPbI3 single crystals stabilizes the phase with the best charge transport characteristics. Both photodetector and J–V measurements suggested that FA0.85MA0.15PbI3 single crystal exhibits suppressed ion migration compared with the counterpart FA0.15MA0.85PbI3 single crystal. These results represent an important step to highlight the role of organic cations in hybrid perovskite materials, which will further benefit fundamental understanding of materials and device optimization.


 Please wait while we load your content...
Please wait while we load your content...