Room-temperature methane gas sensing properties based on in situ reduced graphene oxide incorporated with tin dioxide
Abstract
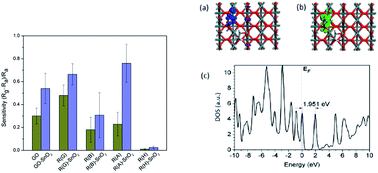
We report on the relationship between the degree of reduction of graphene oxide (GO) and its room-temperature methane gas-sensing response by comparing four in situ reducing agents of GO: D-glucose, sodium borohydride, L-ascorbic acid and hydrazine hydrate. We found that gas sensing based on D-glucose and L-ascorbic acid had a higher gas response than that based on sodium borohydride and hydrazine hydrate because the residues contained oxygen functional groups. The poorly conductive GO was successfully reduced in situ by L-ascorbic acid to achieve high electrical conductivity and a high methane gas response. The incorporation of tin dioxide (SnO2) into the reduced GO (RGO) further increased the gas response by the p–n junction effect. The heterostructure of L-ascorbic acid-reduced RGO–SnO2 had the highest increase in methane response due to the synergistic effect between dehydroascorbic acid and the SnO2 surface. This was inferred from density functional theory calculations with self-consistently determined Hubbard U potentials (DFT+U). Compared with the current room-temperature methane sensing and fabrication technologies, the sensing technology reported here is cheaper to produce and more environmentally friendly while retaining the best sensitivity and wider sensing range.


 Please wait while we load your content...
Please wait while we load your content...