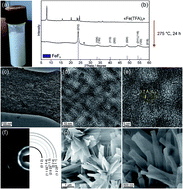
Nanocrystalline FeF3 and MF2 (M = Fe, Co, and Mn) from metal trifluoroacetates and their Li(Na)-ion storage properties†
Abstract
With demands placed on batteries constantly increasing, new positive electrode materials with higher energy density, satisfactory power density, and long-term cycling capabilities, composed of highly abundant elements with low-cost, are desired. One such low-cost cathodic material is iron(III) fluoride – FeF3. Its theoretical capacity for single-electron reduction, accompanied by the insertion of Li+ ions, is 237 mA h g−1. Herein we present a new synthesis for nanocrystalline FeF3 using inexpensive iron trifluoroacetate as a molecular single-source precursor. We also report an adaptation of this simple chemistry to several transition metal difluorides (M = Fe, Co, and Mn). With FeF3, a high capacity of 220 mA h g−1 was attained at a moderate current density of 100 mA g−1 (∼0.5 C). In addition to high capacity, we see the evidence for high rate-capability. Capacities of up to 155 mA h g−1 were observed with 1-minute (10 A g−1) charge–discharge ramps, and at least 88% of this capacity was retained after 100 cycles. When tested as a sodium cathode, FeF3 exhibits capacities of up to 160 mA h g−1 at a current rate of 200 mA h g−1.


 Please wait while we load your content...
Please wait while we load your content...