Effect of ball milling on the electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3 towards the oxygen evolution reaction†
Abstract
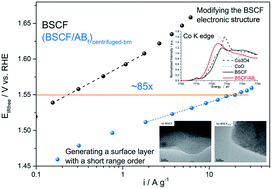
The effect of ball milling and the addition of carbon on the physico-chemical properties as well as the OER performance of the Ba0.5Sr0.5Co0.8Fe0.2O3 (BSCF) perovskite is investigated. We show that the ball milling process can significantly enhance the BSCF OER activity. This higher activity is associated with the formation of an amorphous surface layer produced during the ball milling process, as identified by XRD and TEM. The addition of carbon followed by ultrasonication confirms that carbon acts as a reducing agent to functionalize BSCF but is not necessarily needed during the OER, as identified by X-ray absorption spectroscopy. Our work thus demonstrates that there are at least two different physicochemical methods to enhance the OER activity of BSCF: (i) generating a surface layer with a short range order and/or (ii) modifying the BSCF electronic structure. Further study shows that these two methods can be superimposed to furthermore improve the OER activity of BSCF. The processed BSCF delivers higher mass activity than a benchmark commercial IrTiO2 catalyst, demonstrating the importance of catalyst processing parameters to functionalize highly active OER catalysts.


 Please wait while we load your content...
Please wait while we load your content...