The role of dissolution in the synthesis of high-activity organic nanocatalysts in a wet chemical reaction†
Abstract
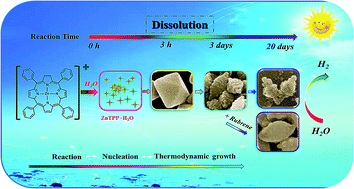
The synthesis of high-activity nanocrystals (NCs) is a key factor in the field of nanocatalysis. By combining nucleation/growth with the dissolution of crystals in a reaction–diffusion system for the first time, we achieved a simple strategy for the one-step synthesis of high-activity uniform nanocatalysts without capping agents (CAs) via simply adjusting the reaction time of the wet chemical reaction (WCR). In this work, the shape evolution of hydrate tetraphenyl-porphyrin zinc (ZnTPP·H2O, ZnP) NCs was systematically studied during the reaction of their precursor with water. Regular, thermodynamic octahedral ZnP NCs can be synthesized at the 3rd hour, and typical rough step-type cuboctahedron NCs can be obtained after 3 days due to the occurrence of chemical dissolution in the multistep WCR. Our results reveal that the crystal dissolution process involves the disappearance of low-energy facets followed by the appearance of high-energy facets. Furthermore, ZnP/rubrene heterojunctions can be easily prepared based on the rough NCs. Compared with regular octahedral ZnP NCs and even nanosheets with more active {020} facets, the rough and heterostructured ZnP NCs exhibit higher performance in photocatalytic hydrogen evolution (PHE). These findings provide a convenient method to synthesize highly active nanocatalysts in a multistep WCR.


 Please wait while we load your content...
Please wait while we load your content...