Rationally tuning host–guest interactions to free hydroxide ions within intertrimerically cuprophilic metal–organic frameworks for high OH− conductivity†
Abstract
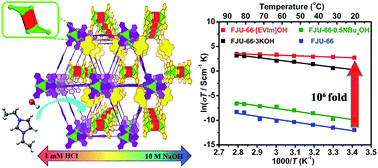
Hydroxide-anion-exchange membrane fuel cells (HEMFCs) are now considered as one of the most promising green energy-conversion technologies for stationary and mobile applications, showing high fuel conversion efficiency at high pH and low cost due to their ability to operate under basic conditions using non-precious metal catalysts. But one key impediment to commercialization is insufficient hydroxide ion (OH−) conductivity of the central HEM component. Here, we report the development of free OH− anion-containing metal–organic frameworks (FOMOFs) with rationally tunable host–guest interactions for HEMs with high OH− conductivity. Among three solids obtained by post-synthesis treatment of ultrastable MOF FJU-66 with various bases, free OH− anions are observed in FJU-66·[EVIm]OH with strong host–guest interactions between the MOF backbone and guest cations. Despite the lowest OH− concentration, FJU-66·[EVIm]OH exhibits the highest OH− conductivity close to 0.1 S cm−1. The high OH− conductivity achieved suggests the potential application of the FOMOFs for practical HEMs of fuel cells.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...