Capacity retention of lithium sulfur batteries enhanced with nano-sized TiO2-embedded polyethylene oxide†
Abstract
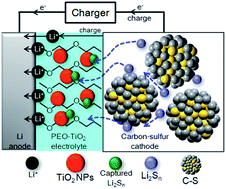
The shuttle effect of polysulfides in Li/S batteries causes the loss of active materials and capacity decay. This phenomenon has retarded the practical application of Li/S batteries. Herein, we demonstrate that the undesired shuttle mechanism owing to dissolved lithium polysulfides can be effectively suppressed by the incorporation of TiO2 nanoparticles (NPs) within a solid nanocomposite polymer electrolyte of polyethylene oxide (PEO). The approach shows enhanced capacity retention, whereby a cell with a hybrid solid electrolyte of TiO2 NPs embedded in PEO has delivered more than 1450 mA h g−1 (first cycle discharge capacity) with ∼87% capacity retention after 100 cycles, compared to only 38% capacity retention in the absence of TiO2. Density Functional Theory (DFT) computational results based on bonding interactions and Raman characterization of the different components reveal that the undesired diffusion shuttle mechanism causing Li anode passivation can be effectively reduced by the use of nano-sized TiO2 and polyethylene oxide.


 Please wait while we load your content...
Please wait while we load your content...