The co-electrolysis of CO2–H2O to methane via a novel micro-tubular electrochemical reactor
Abstract
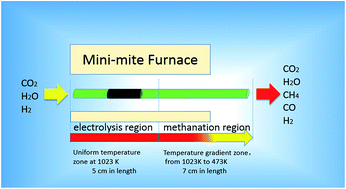
Efficient and direct conversion of CO2 to hydrocarbons through electrolysis is a promising approach for energy storage and CO2 utilization. In this study, high temperature co-electrolysis of H2O–CO2 and low temperature methanation processes are synergistically integrated in a micro-tubular reactor. The temperature gradient along the micro-tubular reactor provides favorable conditions for both the electrolysis and methanation reactions. Moreover, the micro-tubular reactor can provide high volumetric factor for both the electrolysis and methanation processes. When the cathode of the micro-tubular reactor is fed with a stream of 10.7% CO2, 69.3% H2 and 20.0% H2O, an electrolysis current of −0.32 A improves CH4 yield from 12.3% to 21.1% and CO2 conversion rate from 64.9% to 87.7%, compared with the operation at open circuit voltage. Furthermore, the effects of the inlet gas composition in the cathode on the CO2 conversion rate and the CH4 yield are systematically investigated. Higher ratio of H : C in the inlet results in higher CO2 conversion rate. Among all the cases studied, the highest CH4 yield of 23.1% has been achieved when the inlet gas in the cathode is consisted of 21.3% CO2, 58.7% H2 and 20.0% H2O with an electrolysis current of −0.32 A.


 Please wait while we load your content...
Please wait while we load your content...