Spontaneous linker-free binding of polyoxometalates on nitrogen-doped carbon nanotubes for efficient water oxidation†
Abstract
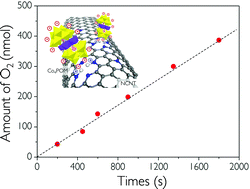
Efficient water oxidation remains a principal challenge for clean fuels via water splitting. Polyoxometalates (POMs) are promising water oxidation catalysts in a neutral medium but their application is commonly limited by low electrical conductivity and poor adhesiveness arising from bulky and electrically insulating ligands. Here we report linker-free spontaneous binding of tetracobalt-based polyoxometalates (Co4POMs) on nitrogen-doped carbon nanotubes (NCNTs) via electrostatic hybridization. Protonated nitrogen-dopant sites at NCNTs enable linker-free immobilization of the Co4POMs and fluent electron transfer in the resultant Co4POM/NCNT hybrid structures, as demonstrated by the low overpotential of 370 mV for the water oxidation at pH 7. Accordingly, the hybrids exhibit fast reaction kinetics with a turnover frequency of 0.211 s−1 at 2.01 V vs. RHE. Density functional theory calculation proposes that POMs vertically align at the NCNT surface exposing the maximal catalytic surfaces. This work suggests a reliable route to highly efficient water oxidation catalysis by employing POMs under neutral conditions and NCNTs as self-binding nanoelectrodes in a synergistic well-oriented hybrid structure.


 Please wait while we load your content...
Please wait while we load your content...