Insights into the electronic bands of WO3/BiVO4/TiO2, revealing high solar water splitting efficiency†
Abstract
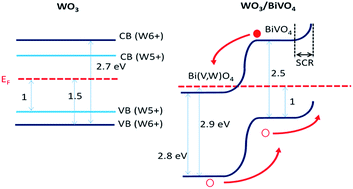
In photoelectrochemical (PEC) water splitting, heterojunction electrodes consisting of two or more dissimilar semiconductors offer more advantages over those made from single semiconductors. Pairing tungsten trioxide (WO3) with bismuth vanadate (BiVO4) is a promising strategy that helps to achieve better charge separation and improved overall performance in PEC water splitting. In this work, we have demonstrated a WO3/BiVO4/TiO2 core–shell nanostructured photoanode for efficient water splitting and stability. A detailed valence band (VB) edge analysis of WO3/BiVO4 was performed with X-ray photoelectron spectroscopy and ultraviolet photoelectron spectroscopy, and it was revealed that due to electronic equilibration between WO3 with BiVO4, Fermi energy level shifting occurs, forming a p–n junction at the interface that allows electrons from the photoexcited BiVO4 to be transferred efficiently to WO3, achieving better charge separation and higher efficiency. This electrode exhibited significantly higher photocurrent density than the individual WO3 and BiVO4 electrodes, producing an unprecedented photocurrent of 4.2 mA cm−2 at 1.23 V versus a reversible hydrogen electrode under simulated sunlight without an added catalyst. A thin layer of photocatalytically inactive amorphous TiO2 was deposited onto WO3/BiVO4 to react with surface defects, deactivating them toward surface charge-carrier recombination, and to increase the stability of photoanodes.

 Please wait while we load your content...
Please wait while we load your content...