Highly active and stable AuNi dendrites as an electrocatalyst for the oxygen reduction reaction in alkaline media†
Abstract
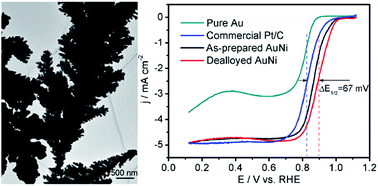
Bimetallic AuNi nanodendrite catalysts have been prepared for the oxygen reduction reaction (ORR) in alkaline media by a facile electrodeposition and electrochemical dealloying method. The dealloyed AuNi catalyst consists of hierarchical dendrites with a high electrochemically active surface area. The half-wave potential (E1/2) of the dealloyed AuNi catalyst is 0.896 V vs. RHE, exhibiting about 67 and 27 mV positive shift relative to the commercial Pt/C and as-prepared (before dealloying) AuNi catalysts, respectively. Compared to the commercial Pt/C catalyst, the dealloyed AuNi achieves a 2.8-fold improvement in specific activity at 0.8 V vs. RHE and suffers less degradation of the ORR activity after 5000 potential cycles. The ORR catalyzed by the bimetallic AuNi catalyst proceeds through a four-electron pathway in basic solution. TEM and XPS characterizations indicate that the enhancement of ORR activity is attributed to the favorable morphology and electronic effect caused by the incorporation of Ni atoms into the Au substrate. Dealloyed AuNi hierarchical dendrites possess great application potential as cathode electrocatalysts in metal-air batteries and alkaline fuel cells due to the facile preparation, high ORR activity and long-term cycling durability.


 Please wait while we load your content...
Please wait while we load your content...