Light-weight functional layer on a separator as a polysulfide immobilizer to enhance cycling stability for lithium–sulfur batteries†
Abstract
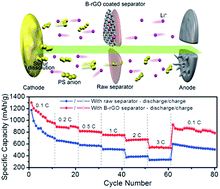
A light-weight boron-functionalized reduced graphene oxide (B-rGO) layer (only 0.2–0.3 mg cm−2) coated on a separator is demonstrated to improve the cycling stability and rate performance of lithium–sulfur batteries. Such an enhanced performance is ascribed to: (i) the boron species in B-rGO can enhance the binding with polysulfides, which helps suppress the shuttle reactions, thus alleviating overcharge and self-discharge; (ii) a certain amount of the boron doped into the graphene matrix can improve the electrical conductivity of the coating layer, thus enhancing the utilization of sulfur and improving the rate performance of the cells. With the B-rGO coated separator, the severe self-discharge of Li–S batteries can be alleviated. More importantly, for the high sulfur loading cathodes (above 4.5 mg cm−2), an improved high areal capacity of 4.71 mA h cm−2 can be achieved using the B-rGO coated separator. The above results demonstrate the potential of the B-rGO coated separator for practical lithium–sulfur batteries, and such a strategy can be extended to other energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...