Using confined carbonate crystals for the fabrication of nanosized metal oxide@carbon with superior lithium storage capacity†
Abstract
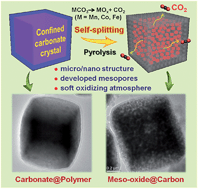
Carbon coating of metal oxides is an effective approach to prepare anode materials for LIBs. However, during high-temperature pyrolysis, metal oxides can be easily converted to their metallic phase by carbothermal reduction and tend to form big particles. A new method proposed for the preparation of meso-oxide@carbon is the pyrolysis of polydopamine-encapsulated carbonate crystals. The confined carbonate splits into nanosized particles and the generated CO2 gases not only create interconnected mesopores throughout the particle and abundant micropores in the carbon layer, but also provide a soft oxidizing atmosphere, which can effectively promise a pure oxide phase by preventing the formation of the metallic phase arising from carbothermal reduction during the pyrolysis of polydopamine. Taking MnCO3 as an example, the MnO@C hybrid consists of nanosized MnO (∼15 nm) with carbon shells (∼18 nm thick) and developed mesopores, which delivers an excellent reversible capacity of 886 mA h g−1 over 200 cycles at 0.2 A g−1. Even at a high current density of 2 A g−1, a superior capacity of 770 mA h g−1 is retained after 300 cycles. This novel approach also offers an effective preparation of other materials such as CoO/Co3O4@C and Fe3O4/γ-Fe2O3@C, which respectively show reversible capacities of 1058 and 770 mA h g−1 at 0.2 A g−1, with good cycling stability.

 Please wait while we load your content...
Please wait while we load your content...