Multi-ionic lithium salts increase lithium ion transference numbers in ionic liquid gel separators†
Abstract
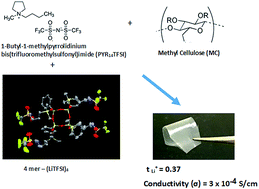
Solid ion-gel separators for lithium or lithium ion batteries have been prepared with high lithium ion transference numbers (tLi+ = 0.36), high room temperature ionic conductivities (σ → 10−3 S cm−1), and moduli in the MPa range. These were formed from the room temperature ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide (PYR14TFSI), a polysilsesquioxane multi-ionic lithium salt that contains four phenyl groups and four pendent LiTFSI (4mer-(LiTFSI)4) on a SiO1.5 ring, and methyl cellulose (MC). After co-dissolution in dimethyl sulfoxide (DMSO) and evaporation of the DMSO, ion-gels were formed at different PYR14TFSI/MC ratios but at constant 0.20 M 4mer-(LiTFSI)4. Conductivity decreased but tLi+ increased with increased MC content. Differential scanning calorimetry, dynamical mechanical analysis and X-ray diffraction data indicated that the PYR14TFSI/MC/4mer-(LiTFSI)4 ion gels were micro-phase separated into a conductive PYR14TFSI/4mer-(LiTFSI)4 phase and one in which the MC was swollen with PYR14TFSI/4mer-(LiTFSI)4. The high tLi+ was attributed to the large size of the anion, its decreased ability as the result of its rigid structure to form ion aggregates, hydrophobic/hydrogen bonding interactions of the 4mer-(TFSI−)4 anion with MC, and participation of the MC hydroxyl groups in the solvation sphere of Li+, weakening its interaction with the 4mer-(TFSI−)4 and TFSI− anions. The high moduli were the result of the preserved semi-crystalline, high glass transition (Tg), fibrillar structure of MC in the ion gels.

 Please wait while we load your content...
Please wait while we load your content...