Ab initio investigations on bulk and monolayer V2O5 as cathode materials for Li-, Na-, K- and Mg-ion batteries
Abstract
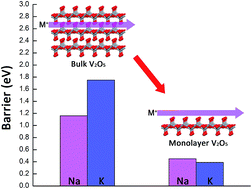
First-principles computations based on density functional theory (DFT) were performed to investigate the performance of bulk and monolayer V2O5 as the cathode material for Li-, Na-, K- and Mg-ion batteries. Both the average voltage and ion migration barrier were studied. The results indicate that alkali metal ions with a large ionic radius (such as Na and K) have much lower migration barriers (0.44 and 0.39 eV for Na and K, respectively) on monolayer V2O5 than in bulk V2O5 (1.17 and 1.66 eV) without great voltage loss, while for Li polymorphs, the difference between monolayer and bulk V2O5 is minimal. However, the performance of monolayer V2O5 is not ideal enough as the cathode material for multivalent metal-ion (such as Mg) batteries. As a result, for Na- and K-ion batteries with a large ionic size, monolayer V2O5 is an attractive cathode material.


 Please wait while we load your content...
Please wait while we load your content...